Clinical Trials Centre
The Clinical Trials Centre at Wesley Research Institute has been managing trials since 1994. Clinical trials are a type of research involving human volunteers dedicated to offering new and emerging treatment options to patients with various illnesses and diseases.
About the Clinical Trials Centre
The purpose of a clinical trial is to evaluate new approaches in treating medical conditions or diseases under controlled conditions. This involves determining how well people respond to new treatments, what side effects there are, how well they are tolerated or whether the new intervention is better than those that are already available.
The conduct of high-quality clinical trials is not only essential in the development of new medicines and medical devices, but they are also necessary to find new uses for existing medicines.
The Clinical Trials Centre enables patient participation in national and international phase I-IV clinical trials with a focus on evaluating new therapies, drugs, and diagnostic tools to drive discoveries into standard clinical practice.
Engaged across four hospitals – The Wesley Hospital, St Andrew’s War Memorial Hospital, Buderim Private Hospital and St Stephen’s (Hervey Bay) – clinicians and patients located in both metropolitan and regional areas can participate.
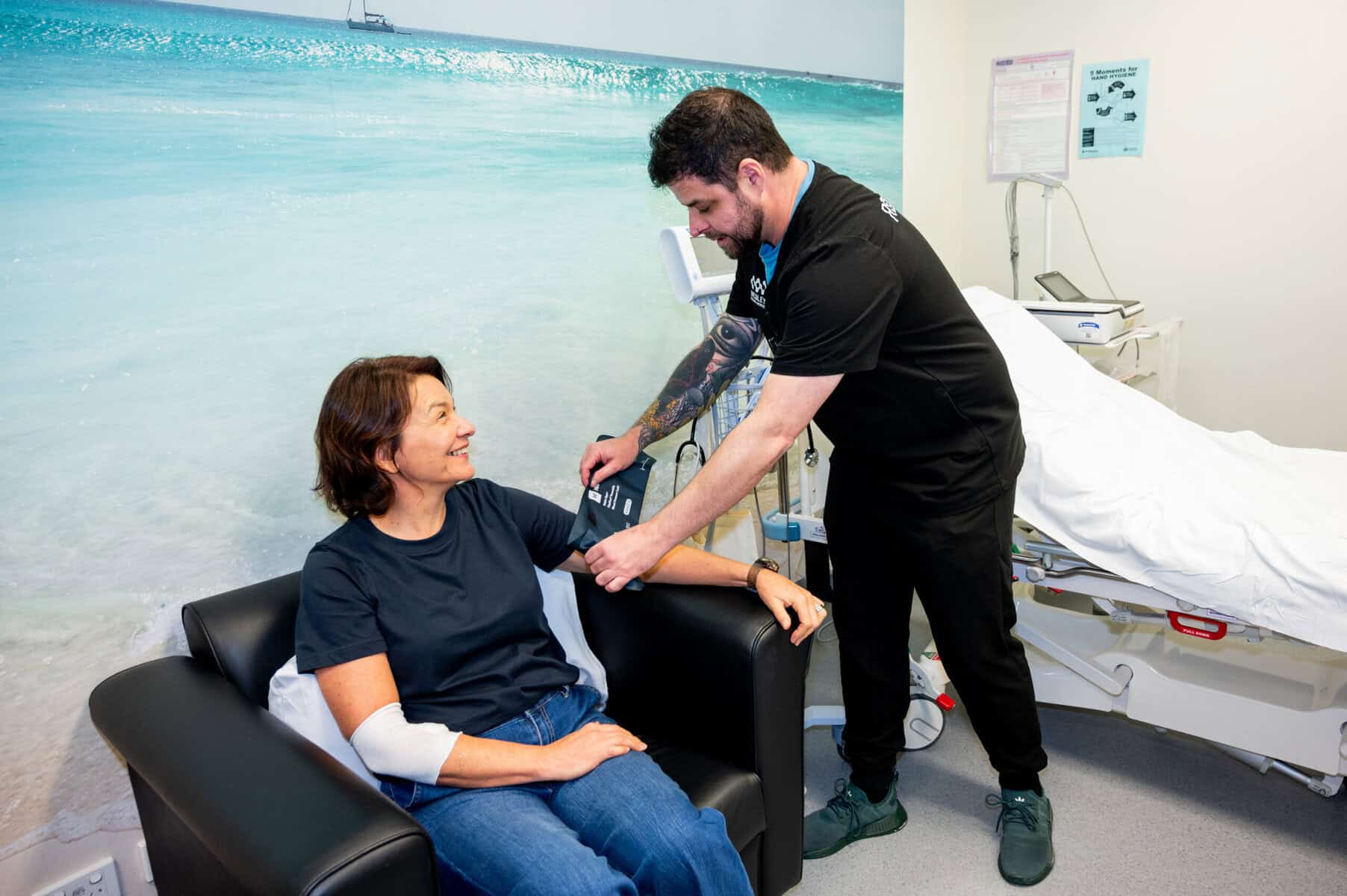
Our patients are at the heart of all we do and that’s why Wesley Research Institute focuses on providing participants, researchers, and sponsors with a first-class, professional experience. Our dedicated staff strive for a reputation of integrity and quality, delivering a warm, caring and inviting environment for all participants.
What clinical trials mean to participants
Clinical trials at Wesley Research Institute

Coeliac Disease Clinical Trials
MOZART

FB102

ARODUX

CONVERT II

APEX

Vertex

INSIGNIA

PROSERA

Chugai
Contact Us
For more information or if you would like to discuss a new Clinical Trial please contact our team:
Email: clinicaltrials@wesleyresearch.shoredigital.com.au
Phone: 07 3721 1500
Facilities
Wesley Research Institute is swipe card access only and is located on the 8th Floor of the East Wing of The Wesley Hospital.
It offers the following facilities for clinical trials:
- Four clinical consultation rooms
- Pathology Laboratory (including 2x vaccine refrigerators with back to base alarms and 24/7 temperature monitoring)
- Dedicated drug safe
- Two Patient Observation Lounges
- Treatment Room
- 3 x -80˚C freezers
- -20˚C freezer
- Refrigerated centrifuge
- 3 x ambient centrifuges
- Secure document storage
- Ample monitoring space